Oxbridge-Science-Academy.github.io
Differentiating between similar chemical groups
We have now fomalised our understanding of why different bonds absorb radiation of different frequencies. This allows us to identify functional groups in a molecule which helps in structure determination.
The stiffness of the bond is related to its strength – a stronger bond will be stiffer and oscillate a higher frequency. While the biggest factor determining the bond strength is the overlap of atomic orbitals of the two atoms directly bonded together, nearby atoms can also make a difference. This can be done in several ways. Electronegative atoms can withdraw electron density from a one of the bonding atoms, changing the energy and size of the atom’s atomic orbitals, affecting the bond strength. Conjugation where a molecular orbital stretches over 3 or more atoms can also affect matters.
High resolution IR spectroscopy can thus be used to probe bond strength in many areas of Chemistry and indeed some current research involves measuring IR absorption frequencies in real time to track the progress of a reaction. For example, as carbon monoxide adsorbs to a metal surface, interactions with the metal electron density changes the CO carbon oxygen bond strength and so changes the IR absorption frequency.
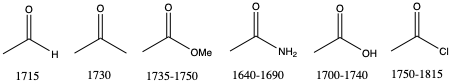
This sensitivity means it is possible to differentiate between very similar groups such as esters and amides. Both have a carbonyl bonds so will absorb at very similar frequencies. However, the oxygen of the ester and nitrogen of the amide will change the strength of the carbonyl bond by different amounts.
In esters, the sp3 oxygen withdraws electron density (inductive effect), making the carbonyl carbon more 𝛿+ and increasing the polarity and thus the bond strength. This affect outweighs the conjugative effect where the oxygen lone pair is delocalised into the carbonyl 𝜋 bond, reducing the bond strength. The net result is a stronger bond.
In amide, the sp3 nitrogen also withdraws electron density (inductive effect), making the carbonyl carbon more 𝛿+ and increasing the polarity and thus the bond strength. However this affect is outweighed by the conjugative effect – the nitrogen’s lone pair is higher in energy than ester’s oxygen and better able to delocalise into the system. The net result is a weaker bond.
We would therefore expect the absorption of ester to be higher in energy than for an amide and this is indeed observed: 1735−1750 cm-1 for an ester and 1640−1690 cm-1 for an amide.
Carbonyls
X-H
C-H 2900−3200 cm-1
O-H 3500−3600 cm-1 (sharp, no H bonding)
O-H 2900−3500 cm-1 (broad, H bonding)
N-H 330cm-1
Assigning IR Spectra
As an undergraduate you will be required to assign IR spectra – some of which will be pre-prepared in the form of the questions and some will be your own spectra recorded from products made in synthetic chemistry experiments. Assigning in this context means stating which bond stretch is responsible for every peak over 1500 ccm-1 . The region below 1500 cm-1 is known as the footprint region and is much harder to analyse without a computer due to overlapping peaks and other factors which are beyond the scope of this course.
Certain bond stretches produce board peaks and other bond stretches produce very sharp peaks. Information like this should be commented on in the assignment.